Abstract
Hypomethylating agents (HMA) are the current standard care for MDS and CMML. However, a large proportion of patients experience HMA treatment failure that is associated with poor prognosis. Novel treatments are urgently needed for these diseases. We performed next generation sequencing (NGS) based mutation profiling that targeted the 81 most frequently mutated genes in baseline bone marrow (BM) mononuclear cells (MNCs) from patients with MDS and CMML (N=83) prior to HMA treatment. Analysis of impact of the mutations on outcomes of HMA treatment indicated that patients with TP53 mutations (N=22) had significantly worse overall survival (HR = 3.29; P < 0.05; q < 0.05). This result is consistent with previous reports and suggests that strengthening the activity of wild type (WT) TP53, through strategies such as inhibition of TP53 negative regulator MDM2, may improve therapeutic efficacy of HMAs in patients with MDS and CMML.
To test this hypothesis, we evaluated in vitro the effects of the combination of the MDM2 inhibitor DS-3032b and azacytidine (AZA). Due to the dearth of MDS or CMML cell lines, initial analysis was performed in the TP53 WT AML cell line MOLM13. Results indicated that DS-3032b and AZA combination induced a synergistic decrease in cell survival (coefficient of drug interaction=0.65), accompanied with increased apoptosis and CDKN1A1 (P21) RNA expression. No combination survival effect was observed in the TP53 mutant AML cell lines SKM1 or TF1.
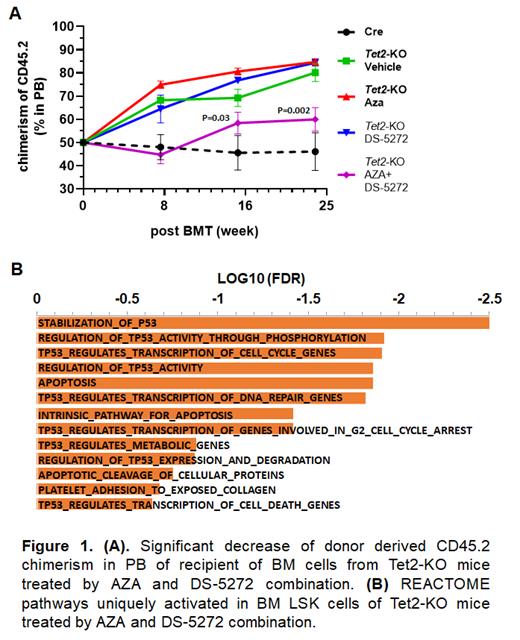
We next evaluated in vivo combination therapeutic effects of the MDM2 inhibitor DS-5272 and AZA in a Tet2-knockout (KO) (Tet2flox/flox/Vav-Cre) mouse model. Prior work indicated that Tet2-KO mice had an MDS/CMML-like phenotype and a persistent long-term (LT) BM repopulating activity that was resistant to AZA. DS-5272 and AZA were administered to mice sequentially: DS-5272 (50 mg/kg) was administered orally for 5 days, followed by daily AZA (2.5 mg/kg, i.p.) for a 7 days. DS-5272 was also administered on the 1st and 3rd days during AZA treatment. After a 2-week gap, the treatment cycle was repeated once. Following the combination treatment, Tet2-KO mice showed significantly reduced monocytosis (P<0.05) and increased platelet counts (P<0.001) in peripheral blood (PB), and significant reductions in Gr1+/CD11b1+ myeloid cells (P<0.001), common myeloid progenitors (CMPs) (P<0.005), and Lin-/Sca1+/c-Kit1+ cells (LSKs) (P<0.01) in BM. Next, we evaluated the impact of treatment on LT BM repopulation activity. CD45.2+ BM cells isolated from drug-treated Tet2-KO mice were mixed with BM cells from CD45.1 WT mice and then transplanted into lethally irradiated CD45.1 WT recipient mice. Significantly lower (P<0.05) CD45.2 chimerism was observed in PB of the recipients transplanted by BM cells from DS-5272 and AZA combination treated Tet2-KO mice compared to recipients that received cells from vehicle control and mono-agent treated Tet2-KO mice, starting from 4 months post-transplantation (Fig.1A). Lower CD45.2 chimerism was also observed in the BM LSK population (P<0.001) of the same recipients at the endpoint of 6 months post-transplantation.
To study the molecular mechanisms underlying the combinational therapeutic effects of the MDM2 inhibitor and AZA observed in the Tet2-KO mice, we performed RNA-seq on BM LSK cells isolated from drug-treated mice. Compared to LSKs from vehicle control mice, over 800 genes with significantly altered RNA expression (>1.5 fold; FDR<0.05) were identified in the LSKs from DS-5272 and AZA combination treated Tet2-KO mice, whereas 110 and 476 genes with altered RNA expression were identified in the LSKs of DS-5272 or AZA treated Tet2-KO mice respectively. REACTOME pathway analysis identified 263 pathways that were significantly (FDR<0.25) and uniquely activated in the BM LSKs from mice treated with DS-5272 and AZA combination. Many of these pathways were associated with TP53 stability, TP53 activity, and TP53 regulated apoptosis and cell cycle (Fig.1B), suggesting a coordination between MDM2 inhibition and HMA treatment to activate TP53 in BM HSPCs.
In conclusion, our work provides proof-of-concept evidence that combining an MDM2 inhibitor with AZA can improve the therapeutic efficacy of AZA in MDS and CMML, through mechanisms including synergistic activation of TP53 in BM HSPCs and inhibition of LT BM repopulating activity. This concept should be further evaluated through pre-clinical studies and clinical trials.
Wei: Daiichi Sanko: Research Funding. Daver: Amgen: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Hanmi: Research Funding; Trillium: Consultancy, Research Funding; Glycomimetics: Research Funding; Genentech: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Novimmune: Research Funding; FATE Therapeutics: Research Funding; Trovagene: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal